Worthwhile Cancer Documentary
- Dr. Thomas J. Lewis

- Jun 9, 2024
- 3 min read
As Dr. Thomas Levy states, all deterioration is the loss of electrons, and all healing is the gain of electrons.
Our immune system takes electrons away from pathogens. That is the primary mechanism by which it works. Thus, treating something like cancer or infections that can lead to inflammation and cancer with redox agents that can steal electrons is an important approach. In my talks on energy medicine, I explained the concept of electromotive potential. It is the "oomph" of that redox agent. Not all redox agents are created the same. More appropriately, all redox agents are created and function differently.
Two considerations become important.
oomph of the redox agent
pharmacokinetics of the redox agent (can it get to the appropriate location)
What is the redox potential of the tumor or the infection causing that tumor?
Answer: Exactly! What I mean by that is no one is measuring that.
Since the loss or gain of electrons is so fundamental to health and disease - shouldn't we be measuring this? One poor way to measure this is the ESR blood marker. It's a poor measure but better than essentially all others. However, it only measures the redox potential of cell membranes.
So what is a mother - or doctor - to do? Can you guess?
When someone is suffering from a disease like cancer, the answer is to bombard them with treatment with different pharmacokinetics and redox potential. And, try to use agents that either do no harm or have a favorable risk/benefit ratio.
I am currently treating a few people with C. One particular case is a lady, a Ph.D., given 6 weeks to live when I met her and her husband 3 months ago. An update is that the tumor has stopped growing, and her blood markers are extraordinarily better. Noteworthy, she is doing much better physically / symptomatically. In cancer, there are no guarantees. Even though the labs are better - the tumor can trump that.
When it comes to treatments like Brownstein, Mega IGG 2000, doxycycline, and ivermectin, we don't really know their redox potential. All we have is anecdotal and objective evidence about treatments and changes in lab values. When it comes to human health, that has to be good enough.
When we use molecules that absorb light and then perform redox reactions, we actually can "know" the redox potential.
Here is the redox potential for two substances. One is methylene blue, and the other is what I will call SPDT. It's a molecule that I and three other colleagues developed.
Methylene blue light absorption characteristics.
SPDT light absorbance characteristics.
Do not be misled by the Y-axis absorbance scales. It all depends upon the concentration of the solution being measured. It turns out the SPDT has much higher absorbance when all else is equal.
Methylene blue main peak: 664nm, secondary peak 613 nm.
SPDT main peak: 636 nm.
So which one is better? What do you think?
The answer is - BOTH - for someone with cancer because we do not know the redox potential of the tumor - and no doubt it has many redox potentials across the entire tumor.
Here is the assessment of these agents. The 664nm band is a weaker redox agent compared to the 636nm, and 636nm is weaker compared to the 613nm. Also, the 636nm absorbs more strongly - thus making more redox agents available when irradiated with light.
Would you use red light to treat a person with these agents? Think for a moment before looking at the answer.
Answer: Yes, if you don't want to therapy to be very effective, otherwise - NO
Red light only stimulates the 664nm band to develop a redox pair. ORANGE LIGHT (the next big thing - ask me why - or tell me why I'm kidding- I hope) has the energy to create a redox pair with the 636nm and 610nm aspects of the respective molecules.
Note how orange light covers the 613 and 636nm light absorbers.
After red light runs its course and then the next big thing - orange light - runs its course, what will be next?
Clue: ROYGBIV
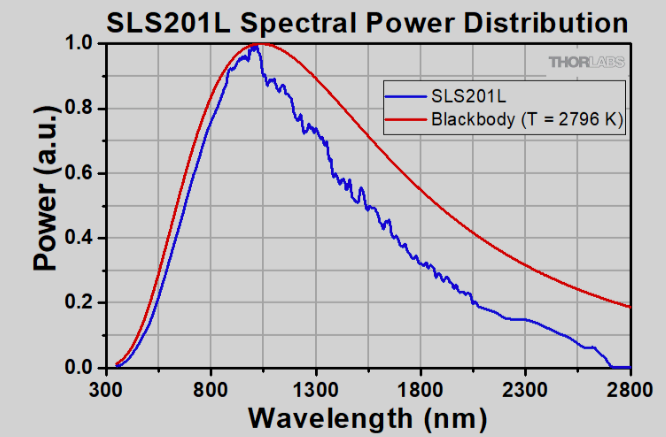
That's why I always use halogen lights, which put out all wavelengths of visible AND a substantial amount of infrared.
Here is the full spectrum of a tungsten halogen light source. NOTE the STRONG amount of light emitted in the infrared. That is why those lights are so darn hot!
Weekly Webinar Links: Join us for detailed health information - at no charge. All are welcome.
Monday at noon EST -
https://zoom.us/j/94642492535?pwd=c2IyOTRoQTdNQ3JhTFdlVXpPMGErUT09
Wednesday at 8 pm EST -
https://zoom.us/j/96863715606?pwd=VTRCNTQ1dEVoWnlRQjRkeGJYRXlSdz09

Be Bold - Be Brave - Stay Well










Comments